DISCLAIMER: This is not medical advice, engage in DIY HRT at your own risk; the intent of this post is harm reduction. This information is not intended for minors; by reading further you agree you are over the age of 21.
DISCLAIMER 2: This is not rigorous scientific research. I conducted this experiment over the course of a work day, carrying out my actual work while trying to not arouse suspicion. I did not have time to create a calibration curve to conduct quantitative analysis. Further, isolating samples and using IR and NMR to identify impurities would increase my chance of being found out by coworkers. I also forgot to collect a data point and made another mistake that I explained in the “results” section that I did not have time to correct. That being said, these results are roughly accurate and I am using them to inform my own DIY HRT.
This is somewhat of a follow-up of this post on DragonOrdnance estradiol enanthate, and I plan on posting every year with stability data for prepared solutions when properly stored (cool, dark, dry). Since I have 10 vials with 7 mL each, I have enough to provide over a decade of stability data. I also have leftover powder which I also plan on testing yearly.
Abstract:
To improve the safety of the DIY transfem community, it is necessary to demonstrate the thermal stability of estradiol esters to ensure DIYers properly sterilize injection vials. Without an autoclave, a higher temperature is necessary to destroy all bacterial endospores that may be present. Estradiol enanthate (EEn) has considerable thermal stability and can be sterilized at home without the fear of decomposing the EEn. At the suggested home-sterlization conditions of 130degC for 30 minutes, no decomposition was detected. Even at 180degC for 1 hour, less than 5% of the EEn decomposed into an unidentified compound. Light exposure also has minimal effect, with no decomposition detected after six months of continuous exposure to artificial light and indirect sunlight. Home sterilization is recommended to avoid infection. However, it is recommended to store both EEn powder and EEn solutions in a cool, dry, dark area to minimize the decomposition of EEn over longer periods of time (EEn powder is shelf-stable for five years and likely much longer). A follow-up study will focus on long-term stability of properly stored EEn powder and EEn injection solutions.
Introduction:
Last week, I saw a post about a comrade who started HRT and mentioned their sterilization method (130degC for 30 minutes). A discussion in the comments included users suggesting that estradiol enanthate (EEn) would start to decompose at 130degC. This surprised me, as I remember seeing somewhere that estradiol ester injection solutions can be sterilized at higher temperatures than an autoclave (121degC) without significant decomposition. This is important because autoclaves are effective not only for the high temperature, but for the elevated pressure as well (~30 psi, or ~2 atm). Therefore, slightly higher temperatures are required to effectively sterilize injection vials. DIYers likely don’t have access to an autoclave or any kind of pressure vessel, so to improve the safety of transfem DIYers, I aim to alleviate concerns regarding the thermal stability of EEn.
I have a considerable stash of EEn powder that I purchased 1.5 years ago (likely over 2 years past its manufacture date) and I have an injection solution I prepared in bulk, originally intending to prepare vials as needed. This bulk solution has not been subjected to heat but has been on my desk at home where it’s been subjected to artificial light and indirect sunlight (light, like heat, accelerates decomposition of EEn). In this experiment, I determined the stability of EEn under various conditions: as a powder and a solution, and after exposure of the solution to light and/or heat.
Methods:
All injection solutions were prepared as 50 mg/mL EEn in MCT oil with 2% (v/v) benzyl alcohol.
The EEn powder, MCT oil, and benzyl alcohol were kept at room temperature in opaque, resealable plastic bags since receiving them 1.5 years ago. The light-exposed injection solution was kept at room temperature in a clear, resealable plastic bag where it was continuously exposed to artificial light and indirect sunlight for 6 months. Two more injection solutions were prepared the same day as this experiment to represent injection solutions that have not been exposed to significant light.
The “light-exposed solution” was analyzed by GC-MS. Then, the solution was sealed in injection vials and the vials were placed in a GC oven at 130degC for 30 minutes. This “light-and-heat-exposed solution” was analyzed by GC-MS.
One of the freshly-prepared solutions was sealed in injection vials and placed in a GC oven at 130degC for 30 minutes. This is the “heat-exposed solution”. The other freshly-prepared solution was transferred to an open test tube and subjected to a 180degC oil bath for 1 hour. This is the “high-heat-exposed solution” and represents the worst-case scenario of a DIYer sterilizing at a temperature far beyond what is necessary.
Results/Analysis:
The chromatogram of the EEn powder suggests this is a high purity sample, as the only peaks are EEn (at 26.4-26.9 minutes) and the MCT oil from previous analyses (Image 1) This EEn powder was analyzed following the analysis of several EEn solutions, and the needle wash was empty, so there were three other peaks that were confirmed to be triglycerides from the MCT oil residue on the needle. There is also a solvent peak (acetonitrile) at ~1-2 minutes. The mass spectrum is consistent with that of EEn (Image 2).
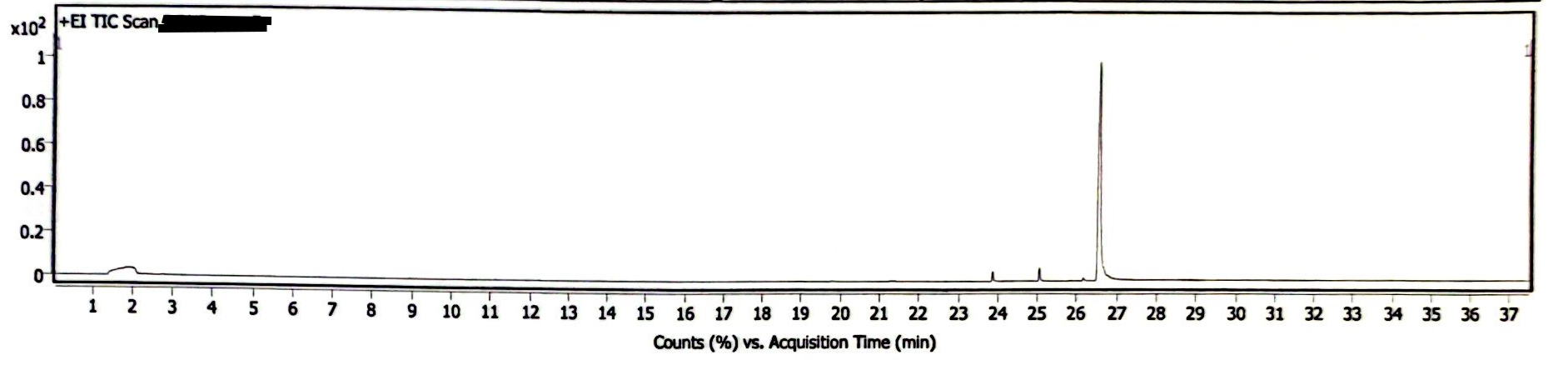
Image 1. GC Chromatogram of EEn powder
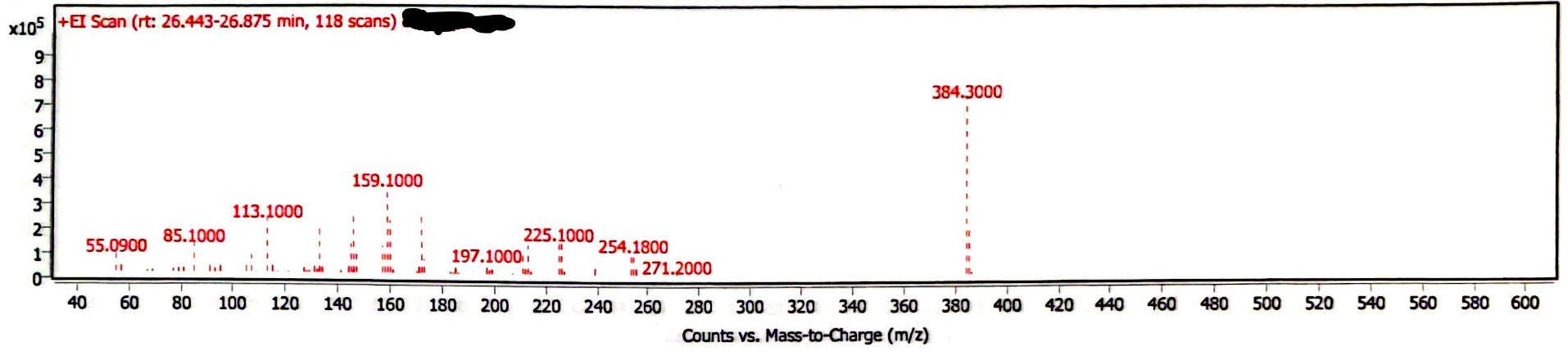
Image 2. Mass spectrum of EEn powder
Image 3 demonstrates a newly prepared EEn solution that was exposed to light and atmosphere while being heated at 180degC for 1 hour. Samples were diluted by adding 1 gram acetonitrile for every 10 mg of sample. As the samples were at the same concentration, the peak area of the EEn peak is a reasonable (though rough) measure of the remaining EEn. Before heating (t= 0), the peak area was 186.0 M counts. At t= 15 min, peak area was 184.9 M counts. At t= 30 min, peak area was 178.7 M counts. At t= 1 hr, peak area was 195.4 M counts. The peak area at 1 hr had the highest z-score at z= 1.5324, giving a p-value of .1254. This indicates it is very likely that the variations in these measurements are due to chance. The chromatogram at t= 1 hr demonstrates the production of a decomposition product, but the peak area of this decomposition product is <5% of the area of the EEn peak (Image 4).
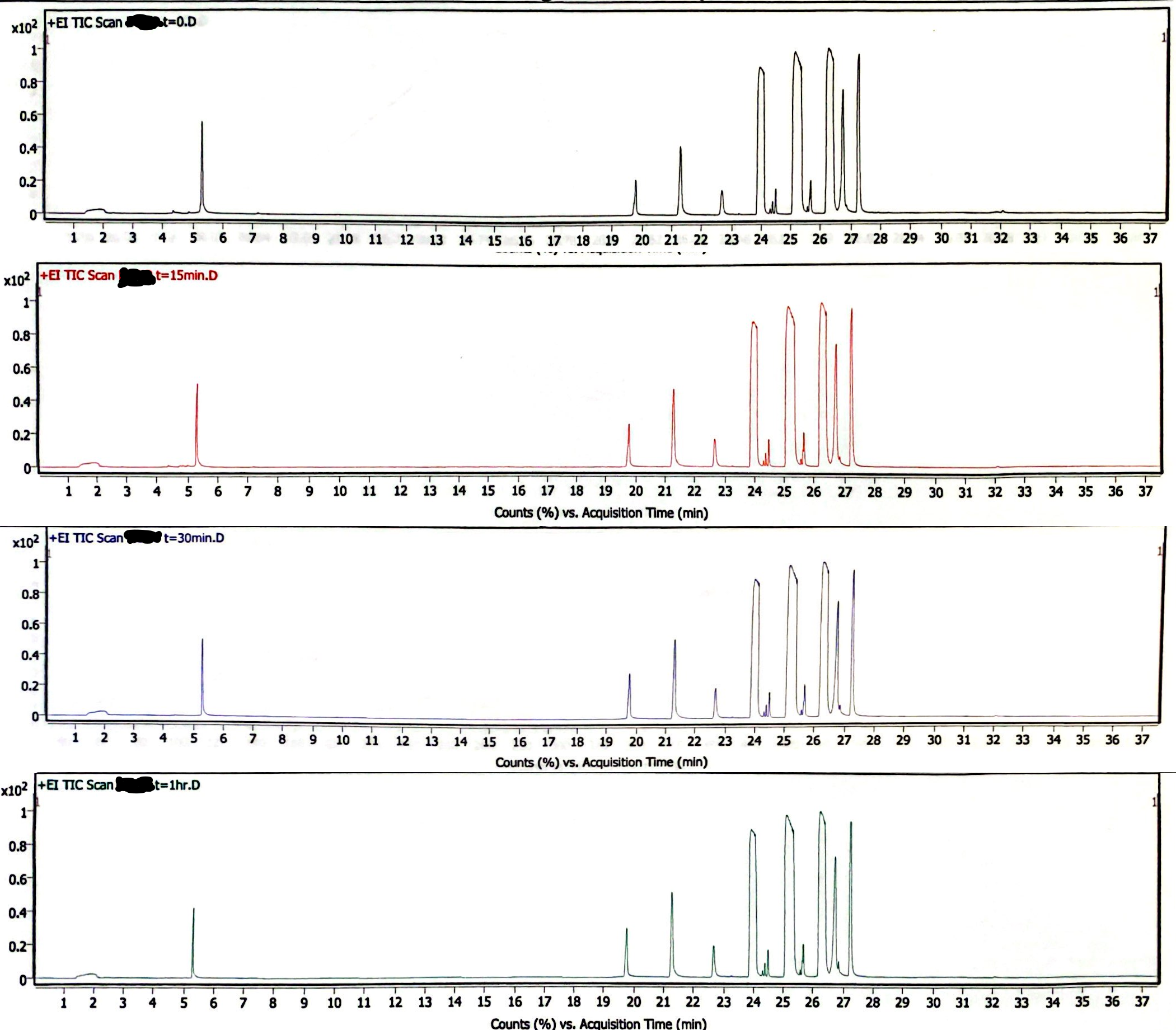
Image 3. High-heat solution chromatograms
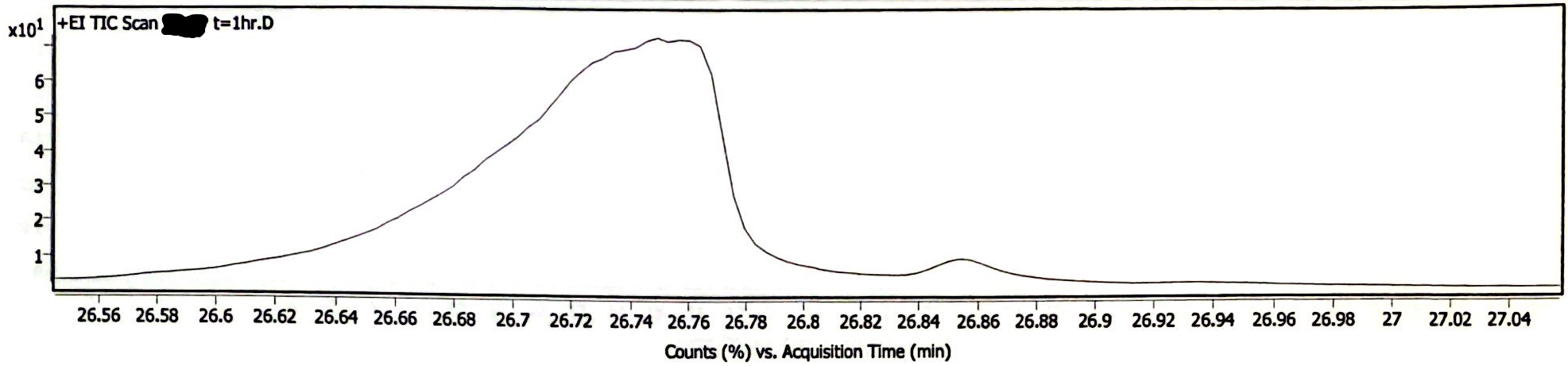
Image 4. High-heat solution chromatogram at t= 1hr, zoomed-in to visualize decomposition product peak
The following three chromatograms represent the light-exposed solution that was not heated (Image 5), the light-exposed solution that was heated at 130degC for 30 minutes (Image 6), and the newly-prepared solution that was heated at 130degC for 30 minutes (Image 7).
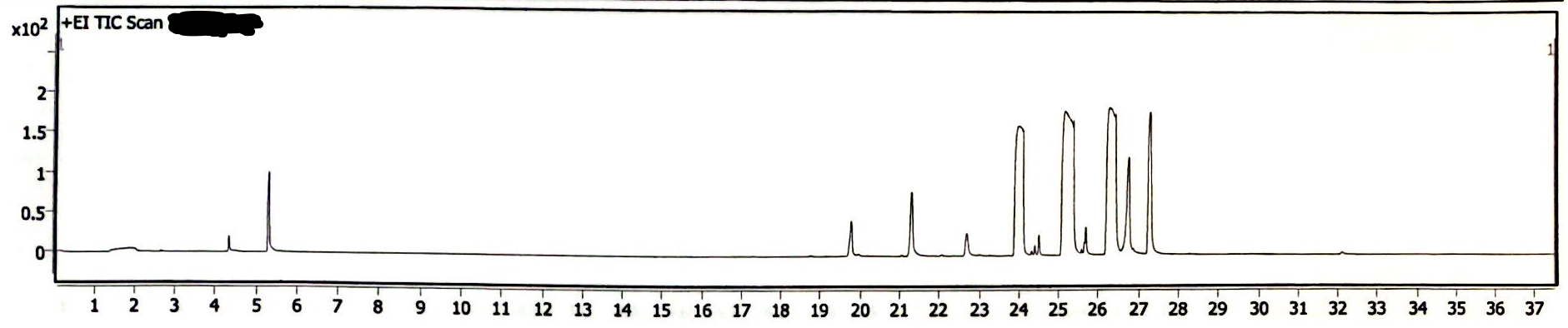
Image 5. Chromatogram of EEn solution exposed to light for six months
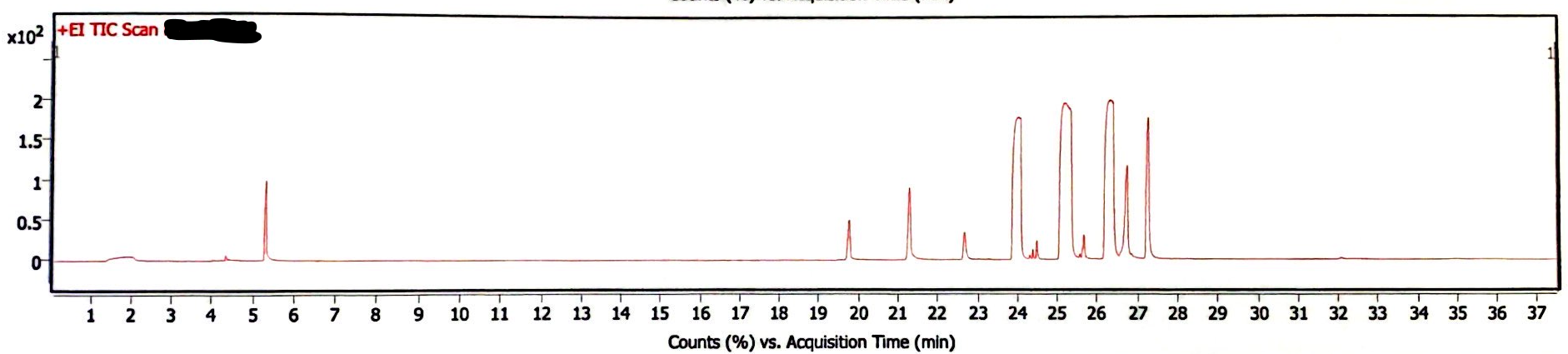
Image 6. Chromatogram of EEn solution exposed to light for six months and 130degC for 30 min
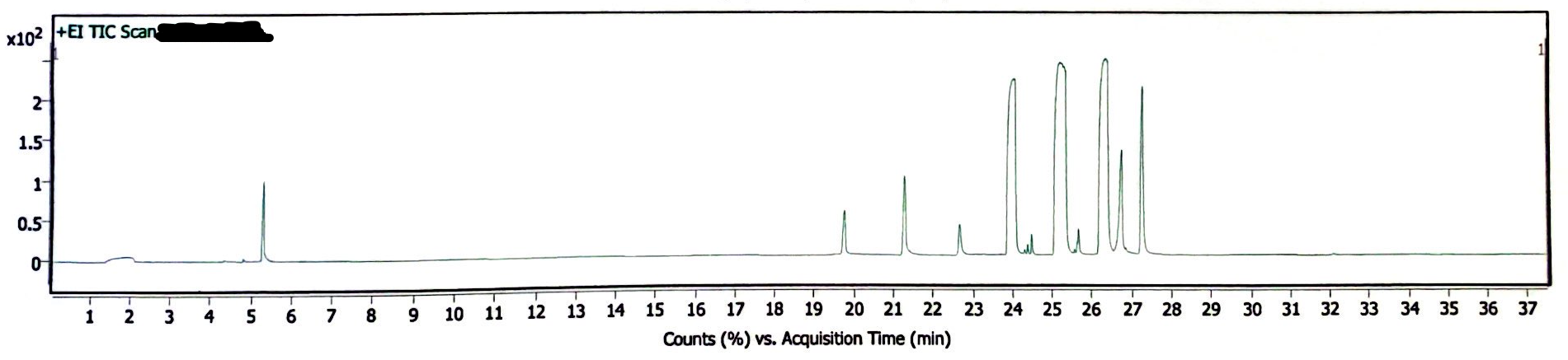
Image 7. Chromatogram of EEn solution exposed to 130degC for 30 min, but no light exposure
In absence of rigorous quantitative analysis, the proportion of peak area of EEn to benzyl alcohol was used to compare the decomposition of EEn. The concentration of benzyl alcohol in these solutions was constant, so it is another reasonable yet rough estimation of remaining EEn to determine if decomposition occured. For the light-exposed/no-heat solution, the EEn peak was 68.99% the area of the benzyl alcohol peak. For the light-exposed/heat-exposed solution, the EEn peak was 68.41% the area of the benzyl alcohol peak. For the no-light/heat-exposed solution, the EEn peak was 68.20% the area of the benzyl alcohol peak. These values are not significantly different, indicating that decomposition did not occur in any of these samples. (I forgot to measure the peak areas of a no-light/no-heat solution, I’m sowwy :(, but you can look at the 180degC data to see that no significant decomposition occurred at 30 minutes at such a high heat).
Conclusion:
According to GC-MS data, no significant decomposition at normal sterilization conditions (130degC for 30 minutes). Even at 180degC, EEn is fairly stable, remaining 95% intact after one hour at this extreme temperature. Light exposure is also a minimal risk, as no significant decomposition occurred after six months of moderate light exposure.
I can verify this user knows their shit and is good

I also crossposted this to blahaj
now someone whip up a latex template for the new journal of traaaaaaannnnnnnnnns research letters
thank you so much for the measurements. this is incredibly helpful.
Yooooo THIS IS AMAZING. THANK YOU. I do DIY mixing for myself and have always wondered whether this decomposed the estrogen. I have regular blood tests done to check my health and I seemed fine, but I'm glad someone has done a review of common methods.
Honestly this is one of the most high effort posts I've seen in any trans forum online
https://transharmreduction.org/hrt-testing
Have you seen this site before? I could refer them to this post and the previous post if youre afraid of sending it to them
Hey! Sorry, I haven't logged in on this account since I made this post. I would really appreciate it if you could share this with them. I am not comfortable doing so myself. Thanks!
Yeah someone sent it in I think though it doesn't seem theyve responded
we wish you had included pictures of the vials themselves... most often its found the vials are discolored after a significant time exposed to light (whether that discoloration actually means anything is a different story)
additionally (when it comes to light exposure) just because the EEn hasnt decomposed doesnt mean the vial itself is safe to use. there is more than just EEn in the vial, and something else is reacting with the light and (could be) turning into something toxic.